
Sentinel is a National Product Monitoring System
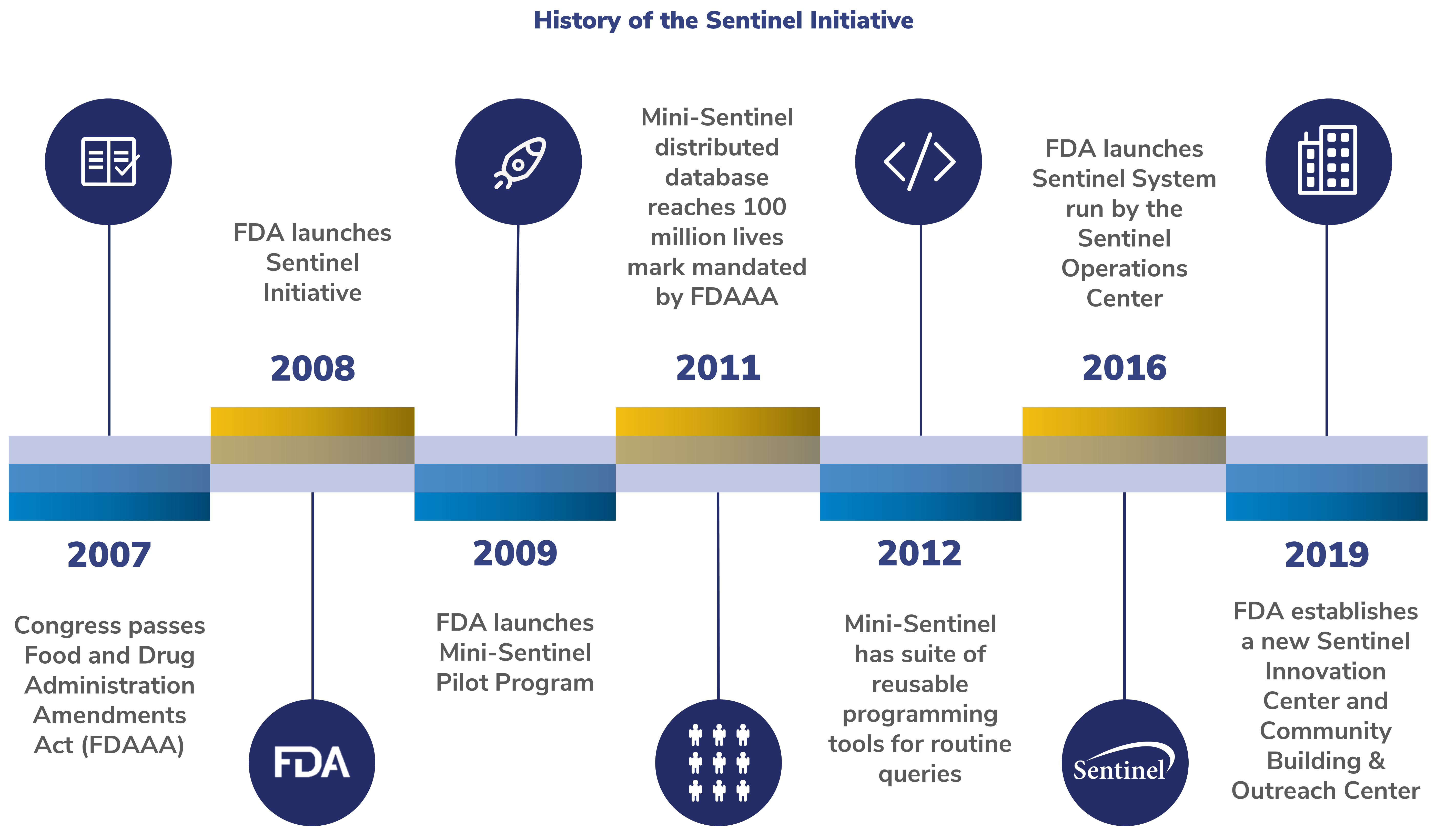
Sentinel is an active surveillance system sponsored by the U.S. Food and Drug Administration (FDA) to monitor the safety of regulated medical products using electronic healthcare data from multiple sources. The Sentinel System is part of the FDA's Sentinel Initiative, a long-term effort to improve the FDA's ability to identify and access medical product safety issues.
The Sentinel Initiative began in 2008 as a multi-year effort to create a national electronic system for monitoring the performance of FDA-regulated medical products. The Initiative is the FDA's response to the Food and Drug Administration Amendments Act (FDAAA) requirement that the FDA work with public, academic, and private entities to develop a system to obtain information from existing electronic health care data from disparate sources to assess the safety of approved medical products.
The Harvard Pilgrim Health Care Institute (HPHCI) leads the Sentinel Operations Center (SOC) and works closely with the FDA to assess the risks and benefits of marketed medical products. The primary functions of the SOC are to:
- Design and conduct medical product utilization, safety, and effectiveness studies in close collaboration with FDA
- Enhance core data sources and evolve the Sentinel Common Data Model
- Develop novel distributed analysis tools
- Further enhance capabilities in signal detection and other areas
- Lead real-world-evidence demonstration projects
The SOC also co-leads the Sentinel Innovation Center (IC). This center focuses on developing innovative methods to further advance Sentinel. The primary functions of the IC are to:
- Improve capabilities of Sentinel by leveraging new data sources
- Build tools needed to enable Sentinel queries to include electronic health record data
- Enhance real-world evidence capabilities through advanced analytics
Related Links
- Sentinel Initiative Website
- Sentinel Initiative - Assessments
- Sentinel Initiative - Publications & Presentations
- FDA’s Sentinel Initiative